The Barriers to CAR T-Cell Therapy Success in Prostate Cancer - 053
Doctors and researchers are studying chimeric antigen receptor (CAR) T-cell therapy in advanced prostate cancer. CAR T-cell therapy is a form of immunotherapy, and sadly, immunotherapy of all types is ineffective in the majority of men with advanced prostate cancer.
What is CAR T-cell therapy?
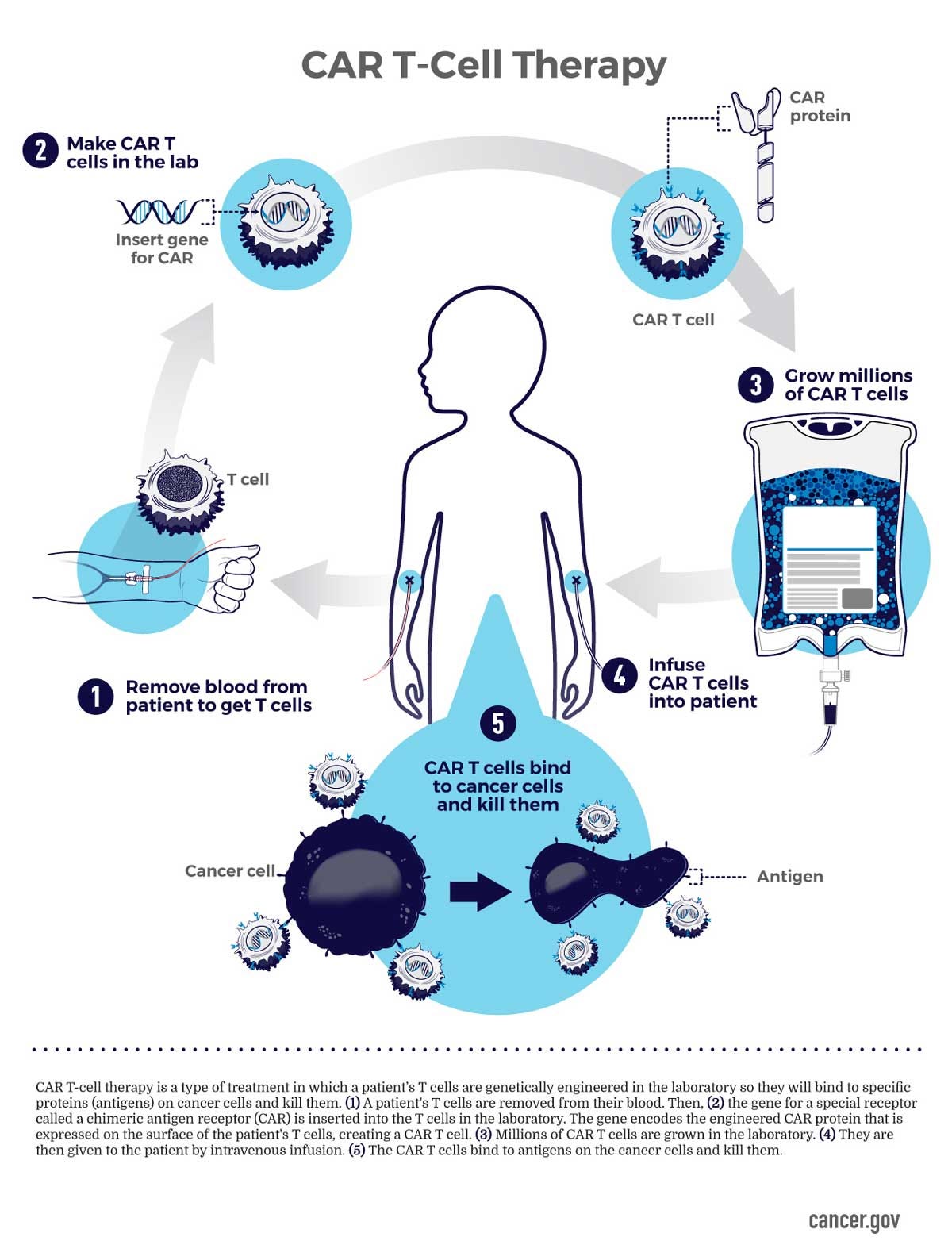
CAR T-cell therapy is a costly cancer treatment that turns immune cells into cancer-fighting machines. It modifies the body's T-cells to recognize tumor-associated antigens and attack cancer cells.
How CAR T-cell therapy works
Researchers collect T-cells (a type of white blood cell) from the blood.
Labs genetically engineer them to express chimeric antigen receptors that target cancer cells.
These genetically engineered cells are grown into millions.
Doctors reinfuse these cells back into the body.
The CAR T-cells then seek out and destroy cancer cells expressing the specific antigen the T-cells were genetically engineered to target.
CAR T-cell therapy commonly targets two prostate tumor-associated antigens:
prostate stem cell antigen (PSCA)
prostate-specific membrane antigen (PSMA)
I've previously discussed PSMA, a protein on the tumor cell surface, which is used to detect prostate cancer with PET scans and to treat it with PSMA radioligand therapy (lutetium-177–PSMA-617).
Obstacles of CAR T-cell therapy in prostate cancer
CAR T-cell therapy successfully treats blood cancers because CAR T-cells have direct access to malignant blood cells. However, it faces significant obstacles when treating solid tumors like prostate cancer because of a more complex tumor microenvironment.
The prostate tumor microenvironment creates physical and biochemical barriers that impede CAR T-cell infiltration and function. Prostate cancer often develops dense extracellular matrices within bone or soft tissue that physically block T-cell penetration.
In addition, the prostate tumor microenvironment contains numerous immunosuppressive factors and cells that can deactivate or exhaust CAR T-cells before they can attack cancer cells.
Prostate cancer heterogeneity
Prostate cancer cells also exhibit antigen heterogeneity (diversity), which makes it difficult for CAR T-cells to recognize and effectively target all tumor cells. Antigen heterogeneity refers to the variable and inconsistent expression of target antigens within tumor tissues, which changes as the disease and treatment progresses.
Different regions within the same tumor may show varying levels of antigen expression, such as PSMA. Some areas may completely lack antigen expression, while adjacent areas may express it strongly.
Antigen expression levels vary even among tumors classified with the same Gleason score.
Lack of persistence of CAR T-cells
In phase 1 prostate cancer trials, CAR T-cells show limited persistence in the body beyond 28 days. Only one patient in all trials showed a maximum 8-month response. This lack of persistence contrasts with blood cancers, where CAR T-cells can persist for more than four years in some cases.
Why use CAR T cell therapy for prostate cancer?
Based on the known obstacles to treating prostate cancer with CAR T-cell therapy and the results of phase 1 clinical trials, I can't understand why doctors and researchers are pursuing CAR T-cell therapy research for prostate cancer.
I think that researchers and pharmaceutical companies saw the incredible success of using CAR T-cell therapy for blood cancers and thought they could somehow make it work in prostate cancer. For some, I'd call this delusional thinking based on greed.
The other factor that may be driving some altruistic researchers and doctors is the fact that metastatic castrate-resistant prostate cancer is incurable.
Considering the significant obstacles, risks versus benefits, and the outrageous cost of CAR T-cell therapy, I'd think one would say “no” to allocating resources to studying it in prostate cancer. However, that's not the case, so let's dig deeper into this and try to figure out why.
CAR T-cell therapy in blood cancers
Researchers discovered a gold mine when using CAR T-cell therapy to treat blood cancers. This therapy has shown incredible efficacy in patients with previously untreatable relapsed and refractory blood cancers.
CAR T-cell therapy has astounding response rates in phase 1 clinical trials for blood cancers compared to prostate cancer.
Response rates in blood cancers
Response rates as high as 100% in some hematologic malignancy studies
Complete response rates in 100% of refractory T-cell lymphoma
100% complete response rate for first patient treated with B cell non-Hodgkin lymphoma
92% objective response rate, with 55% complete response rate in multiple myeloma
Now, let's examine response rates in phase 1 clinical trials of CAR T-cell therapy for prostate cancer and prepare not to be amazed.
Response rates in metastatic castrate-resistant prostate cancer
4 of 13 patients (30.1%) achieved ≥30% PSA decline, which did not last
Median PSA decline of 22.35%, which did not last
Best radiographic response was short-lived stable disease in 5 patients (38.5%)
Median overall survival (OS) of 15.7 months, but the study clarifies that all patients were heavily pretreated and most patients went on to receive other types of treatment so the OS cannot be solely due to CAR T-cell therapy
Median progression-free survival (before their disease progressed) 4.4 months
One patient had a PSA decline of > 98% but died due to complications from the CAR T-cell therapy
PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial
Only 4 of 14 patients (28.5%) showed PSA declines >30%, which didn’t last
Maximum response duration of 8 months in a single patient
Limited CAR T-cell persistence in the body beyond 28 days
CAR T-cell therapy response rates in phase 1 trials for prostate cancer are dismal.
The lead investigator of the second trial is a thought leader and expert in prostate cancer. She practices at the City of Hope, a National Cancer Institute-designated comprehensive cancer center.
While researching this newsletter, I found an article titled Trial of CAR-T for prostate cancer takes steps toward 'potentially curative' therapy. In it, she stated, “I really believe these are steps forward toward a treatment that is potentially curative."
At first, I thought she was speaking about City of Hope’s general research on prostate cancer, but then I realized she was talking about CAR T-cell therapy. It is way too early to be throwing around a word like "curative" when talking about CAR T-cell therapy for prostate cancer.
It's very misleading and irresponsible to imply that CAR T-cell therapy will one day be curative for prostate cancer, especially considering the Phase 1 trial results. It is an extreme example of how oncology researchers use phrases like "game changer" and "paradigm shift" when discussing clinical research findings.
Vinay Prasad, M.D. published a research letter in JAMA Oncology titled The Use of Superlatives in Cancer Research.
In our paper, we found that all of the players in cancer drug development - from small biotech companies to industry experts, physicians, and journalists - all contributed to the use of superlatives. At each stage in drug development, there is likely a perverse incentive to embellish one's findings. Small companies want to be sold to larger ones. Trialists want to participate in meaningful research, and journalists seek readers and "clicks." Many forces are aligned to promote the excessive use of superlatives, and there are few countervailing forces to promote accuracy and restraint.
Why might researchers at the City of Hope be so invested in CAR T-cell therapy for prostate cancer that they carelessly use superlatives like “curative?” I’m not sure but I did see in the “Conflict of interest statement” in their study report that the City of Hope owns a patent for chimeric antigen receptors targeted at PSCA.
The risks of CAR T-cell therapy
CAR T-cell therapy is an intensive treatment that often requires inpatient monitoring due to side effects and complications. These risks include cytokine release syndrome (CRS), the most significant treatment-related toxicity caused by rapid immune activation and the release of inflammatory cytokines.
CRS, in its worst form, leads to multiple organ dysfunction syndrome, shock, and death. Even for mild CRS, the treatment is Tocilizumab, an immunosuppressive drug with a wholesale one-dose price of $3683.50.
Another potential side effect of CAR T-cell therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), which can cause seizures and coma.
CAR T-cell therapy requires lymphodepletion with chemotherapy drugs to be most effective. Lymphodepletion clears space for new CAR T-cells by significantly reducing existing lymphocytes and creates a more favorable environment for CAR T-cells to multiply and thrive, but puts patients at risk for infection.
CAR T-cell therapy is a high-cost, resource-intensive, and risky treatment. Speaking of cost, let's look at a cost analysis.
Cost Analysis
The base costs for CAR T-cell therapy are substantial:
Drug acquisition costs: $373,000-$475,000
Hospital/facility costs: $79,466-$85,267
For prostate cancer, these costs become particularly concerning when considering:
Requires lymphodepletion for effectiveness
Possible inpatient monitoring
Management of cytokine release syndrome and other complications
Potential need for multiple treatments due to lack of durability
Finally, based on the phase 1 trial results, let's conduct an economic analysis comparing CAR T-cell therapy for blood cancers to prostate cancer in phase 1 clinical trials. When looking at the numbers, the cost-effectiveness equation becomes particularly concerning.
Economic Analysis
Blood Cancers:
$1 million investment justified by 40-100% complete response rates
Multi-year remissions provide long-term value
One-time treatment is often sufficient
Prostate Cancer:
The same $1 million investment yields a 28.5% partial response rate
Brief duration of response (maximum 8 months in one individual)
The potential need for repeated treatments
Poor persistence requiring other types of additional therapies
Conclusion
The evidence from phase 1 clinical trials strongly suggests that pursuing CAR T-cell therapy for prostate cancer represents a misallocation of healthcare resources.
While the treatment of blood cancers demonstrates cost-effectiveness with durable and complete responses, prostate cancer's biological characteristics appear fundamentally unsuited to this high-priced therapeutic approach.
The combination of extreme heterogeneity, complex immunosuppressive tumor microenvironment, and the lack of persistence of CAR T-cells creates potentially insurmountable barriers that cannot justify the extraordinary costs.
The stark disparity in outcomes compared to blood cancers, combined with the astronomical costs, suggests that continuing to pursue CAR T-cell therapy for prostate cancer may be more driven by the allure of innovation than by rational economic and therapeutic considerations.
Until significant technological breakthroughs can overcome the fundamental biological barriers of prostate cancer, investment in this approach for prostate cancer appears economically unjustifiable.
Researchers might better direct healthcare resources toward more promising treatment modalities. The future of prostate cancer treatment likely lies in approaches that can address its inherent heterogeneity and immune-resistant nature rather than forcing an expensive square peg into a round hole.
Instead of investing billions of dollars in CAR T-cell therapy for prostate cancer, researchers should consider spending that money on developing novel approaches specifically designed for the unique challenges of solid tumors.
I estimate I spent approximately 12-14 hours researching and writing this article. If you like my writing, I’d really appreciate it if you’d consider becoming a paid subscriber.
Until the next newsletter, stay healthy.
Much love,
Keith