Artificial Intelligence and Prostate Cancer - 050
Several FDA-approved artificial intelligence (AI) tools aid prostate cancer diagnosis, including Paige Prostate, an AI-based pathology product, and ProstatID, which combines AI with traditional MRI scanning to improve the speed and accuracy of prostate cancer detection.
But one AI tool I'm most excited about is the ArteraAI Prostate Cancer Test, which helps clinicians stratify risk and help them decide if androgen deprivation therapy is necessary.
Risk stratification
Risk stratification for prostate cancer includes:
How likely is the cancer to be confined to the prostate or spread to regional lymph nodes?
How likely is the cancer to progress or metastasize after treatment?
How likely is adjuvant or salvage radiation to control cancer after radical prostatectomy?
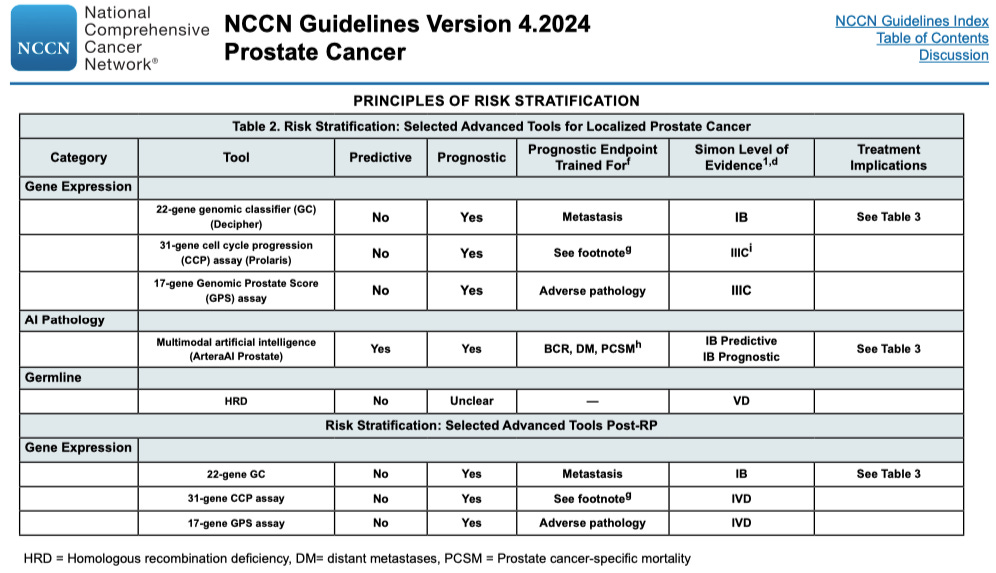
The updated NCCN Guidelines for Prostate Cancer, released on February 27, 2024, include a new table of risk stratification options based on Selected Advanced Tools. These options include gene expression tests, artificial intelligence pathology, and germline testing.
What stands out among these risk stratification models and tests is that the ArteraAI Prostate Test has both predictive and prognostic capabilities with a high IB level of evidence. Most risk stratification models and tests in the NCCN guidelines offer predictive or prognostic stratification, but not both.
Prognostic risk stratification:
Estimates likely outcomes independent of treatment.
Assesses aggressiveness and lethality of the cancer.
Predictive risk stratification:
Evaluates how a patient may respond to a specific treatment.
Identifies which patients are likely to benefit from particular therapies.
Both types of risk stratification aim to personalize prostate cancer management.
A test that is both prognostic and predictive:
Offers a more comprehensive risk assessment.
Allows for more tailored treatment decisions, helping to identify which patients are most likely to benefit from specific therapies.
Helps avoid overtreatment of low-risk patients.
Helps avoid the undertreatment of high-risk patients.
How the test was developed
The ArteraAI Prostate Test for low, intermediate, and high-risk prostate cancer was developed by digitizing histopathology slides of pre-treatment prostate biopsies and studying longitudinal outcomes from five phase III randomized controlled trials. This model included AI learning of 16,204 histopathology slides of 5,564 patients with more than ten years of follow-up.
Treatments given in these five clinical trials included external beam radiation (EBRT) plus or minus different durations of androgen duration therapy (ADT). The goal was to assess the role of multimodal AI in providing prognostication in localized prostate cancer.
The test can be used to decide if treatment is indicated
The data from these five trials showed that the ArteraAI Prostate Test was significantly better than using NCCN risk groups to stratify risk. NCCN stated,
"Given the superior discrimination of the MMAI (Artera AI) model for multiple oncological endpoints over the NCCN risk groups, this test may be used to provide more accurate risk stratification to inform shared decision-making regarding absolute benefit from various treatment approaches."
Daniel Spratt, MD, is Chair and Professor of Radiation Oncology at UH Cleveland Medical Center, Seidman Cancer Center, at the Case Western Reserve University of Cleveland, OH.
Dr. Spratt points out that for localized prostate cancer, doctors traditionally personalize treatment based on prognostic factors, such as the Gleason score, T-stage, and PSA. Those three are not predictive of response to treatments and only estimate the aggressiveness of the disease.
That means doctors may overestimate or underestimate an individual's prognosis and risk of recurrence or metastases and apply therapies to them as a one-size-fits-all approach.
For a patient with intermediate-risk prostate cancer, his Gleason score, T-staging, and PSA level only provide prognostic risk stratification. The ArteraAI Prostate Test also provides predictive risk stratification, which tells you which men will benefit from androgen deprivation.
This is a big deal!
You have all read my rants about androgen deprivation therapy. Despite being the standard of care for over 80 years, it has devastating side effects. Many men with advanced prostate cancer who are treated with ADT go on to develop incurable castrate-resistant prostate cancer due to the mutations induced by the ADT drugs.
We now have high-level evidence of an AI test model that digitizes histopathology slides of a prostate biopsy and can tell you whether or not a man with intermediate-risk prostate cancer is likely to benefit from androgen deprivation therapy (ADT) added to radiation therapy (RT).
"This test is for patients who have been diagnosed with localized prostate cancer who have not yet received radiation therapy or androgen deprivation therapy…"
"The Artera AI Prostate Yest is the first and only artificial intelligence (AI) test that can predict benefit from ADT. For patients with intermediate-risk prostate cancer who are considering adding ADT to RT, this test can predict whether or not they will experience a significant reduction in the risk of distant metastases by adding short-term ADT (ST-ADT) to RT."
"Men with intermediate localized prostate cancer are often treated with short-term androgen-deprivation therapy in combination with radiation therapy (RT + ST-ADT), but only ~⅓ of men actually benefit from it. The AI identifies the 1/3 of patients who may greatly benefit from short-term hormone therapy.'
Short-term androgen deprivation therapy, typically added to radiation therapy, is defined as a duration of four to six months.
More information about the test
It is available through a single CLIA-certified laboratory in Jacksonville, FL.
Clinicians in all states except New York and California can order the test. Artera AI is actively pursuing licensure in those two states.
Results can be shared with clinicians via a portal within 2-3 days after receiving the patient's specimen.
Patients covered by Medicare Plan B should have zero out-of-pocket costs. For patients covered by private insurance, out-of-pocket expenses are determined by the terms of their plan, considering copays, coinsurance, and deductibles.
Sample reports
Go to https://artera.ai/prostate-test-report to view samples of the ArteraAI Prostate Test reports for different risks: very low/low, favorable intermediate, unfavorable intermediate, and high/very high.
The sample reports provide:
A 10-year risk of distant metastases is broken down into high, intermediate, and low risk and given as a specific percentage.
A 10-year risk of prostate cancer-specific mortality given as a specific percentage.
Active surveillance insights - the risk of adverse pathology should a patient undergo a radical prostatectomy. Example interpretation: “This patient has a risk of adverse pathology (AP) at radical prostatectomy (RP) that is lower than 65% of patients who underwent RP in an active-surveillance (AS) - managed cohort. (N=292)”
Clinical interpretation. Example interpretation: “This patient has a 1.8% risk of developing metastasis within 10 years regardless of management with active surveillance, radiation therapy, or radical prostatectomy.”
Prognostic Risk - comparison of this patient to those in the same NCCN risk group. Example interpretation: “This patient has an estimated 10-year risk of metastases that is lower than 51% of patients in the same NCCN very low/low-risk group.”
Intermediate-risk prostate cancer is where this test shines
The sample reports for both favorable intermediate (Gleason 3 +4) and unfavorable intermediate (Gleason 4 + 3) prostate cancer add an "ST-ADT BIOMARKER" section that is either ST-ADT biomarker negative or ST-ADT biomarker positive. ST-ADT stands for short-term androgen deprivation therapy.
ST-ADT Biomarker Negative example interpretation: “On average, patients with this result had no clear risk reduction in distant metastasis with the addition of short-term androgen deprivation therapy.”
"Biomarker negative" means patients with similar pathology slides in those five clinical trials showed no significant reduction of distant metastases within 15 years when treated with radiation therapy plus short-term androgen deprivation therapy compared with radiation alone.
ST-ADT Biomarker Positive example interpretation: “On average, patients with this result had significant risk reduction in distant metastasis with the addition of short-term androgen deprivation therapy.”
"Biomarker positive" means patients with similar pathology slides in those five clinical trials showed a significant reduction of distant metastases within 15 years when treated with radiation therapy plus short-term androgen deprivation therapy compared with radiation alone.
Conclusion
The ArteraAI Prostate Test is an impressive AI pathology tool backed by high-level IB evidence. It lets practitioners and their patients make better-informed decisions about adding androgen deprivation therapy to radiation therapy for intermediate-risk localized prostate cancer.
It also provides "insights into active surveillance," which helps prostate cancer patients make better-informed decisions about proceeding with AS.
We need more high-level AI support in the diagnosis and management of prostate cancer, especially regarding the use of androgen deprivation therapy. This is an excellent start!
I leave for Switzerland in three days to undergo INUSpheresis, and am looking forward to sharing that experience with you.
Until the next newsletter, stay healthy.
Much love,
Keith


Dr. Holden, there is a lot of talk about androgen deprivation and how that slows progression, but if you have a man that has PC contained and is working on his health, losing weight, resistance training etc, these activities will induce a higher testosterone level and does. How does this work in this situation, because the raise in Test is natural, but if T promotes PC growth, what is the advantage of all this? Is there a natural state of Testosterone increase that doesn't progress PC progression? Based on this thought process, I'm inclined to question these tests that are still being built on this deprivation model.
I met with Dr Spratt after reading this article. There is a high likelihood he will be providing my radiation treatment and working with my medical oncologist going forward. Thanks for the referral.