Active Surveillance of Prostate Cancer - 048
Active surveillance (AS) is the standard of care for monitoring low-grade, low-risk, and some favorable intermediate-risk localized prostate cancer. This approach to low-risk prostate cancer started after physicians noticed a pattern of overtreatment of low-risk prostate cancer, resulting in severe side effects from prostatectomy, radiation, and androgen deprivation therapy.
The goal of AS is to monitor for possible progression while preventing or delaying therapy with inherent side effects. Another goal of AS is curative intent - if the patient progresses and needs treatment, appropriate protocols would catch the progression early enough for treatment to result in a cure.
Pioneers of active surveillance
Academic centers like Johns Hopkins, the University of California at San Francisco, and Memorial Sloan Kettering were some of the earliest pioneers of active surveillance in the United States (U.S.). They established studies and protocols in the mid-1990s to generate long-term data that have helped make AS the preferred approach for low-risk prostate cancer that it is today.
AS also has roots in Canada. In 2002, Dr. Laurence Klotz published a prospective active surveillance trial at the University of Toronto, which helped establish its feasibility and safety.
Active surveillance guidelines
Several active surveillance guidelines for prostate cancer are nationally and internationally recognized. They can be found within the following guidelines:
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Prostate Cancer (free login required)
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection (free login required)
National Institute for Health and Care Excellence (NICE), United Kingdom
Michigan Urologic Surgery Improvement Collaborative
Capitalizing on the success of these early adopters of AS is the Michigan Urologic Surgery Improvement Collaborative (MUSIC). Established in 2011 with support from Blue Cross Blue Shield of Michigan, MUSIC aims to improve urological care in Michigan.
The MUSIC AS program publishes its protocol in exquisite detail online, calling it the MUSIC AS Roadmap.
I chose to write about the MUSIC AS Roadmap because it is relatively easy to understand and closely follows NCCN’s guidelines for active surveillance of prostate cancer.
MUSIC AS Roadmap
It splits the protocol into several parts, starting with:
The Consideration Phase: Provides steps to decide whether men are AS candidates.
Step 1: Estimate life expectancy.
Men with a life expectancy of less than ten years enter watchful waiting
Men with a life expectancy of ten years or greater move to Step 2
Step 2: Determines appropriateness for AS based on MUSIC criteria and factors in the Gleason score, tumor volume, PSA density (compares PSA level to prostate size), sexual interest, race, and ethnicity.
Data adapted from MUSIC Active‑Surveillance Placard, 2023
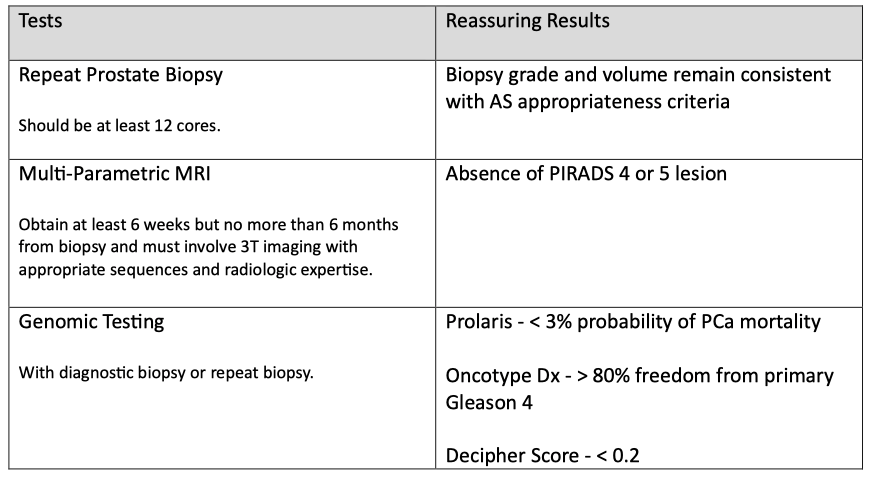
Step 3: Obtain confirmatory testing to evaluate appropriateness.
Sometimes, the initial grade and volume of the tumor are wrong, and this step uses repeat prostate biopsy, multi-parametric magnetic resonance imaging (mpMRI), or genomic testing to increase the confidence that AS is appropriate.
Data adapted from MUSIC Active‑Surveillance Placard, 2023
Step 4: Engage in shared decision-making whether or not to begin treatment or enter the surveillance phase.
Once men enter the Surveillance Phase, practitioners and patients choose either a high-intensity or low-intensity surveillance plan, which varies by frequency of planned follow-up.
Data adapted from MUSIC Active‑Surveillance Placard, 2023
Situations that would prompt further assessment during AS include:
New and concerning digital rectal exam findings
PSA doubling time < 3 years
Other clinical suspicion for disease progression
Patient preference
For more details about MUSIC's AS program, here is a YouTube video that walks you through the roadmap.
Caveat for Gleason 3 + 4 tumors
I don't see where MUSIC's roadmap factors in cribriform growth patterns in men with low-volume Gleason 3 + 4 tumors, but I suspect it does. A study suggests active surveillance programs should consider cribriform growth patterns when selecting Gleason 3 + 4 prostate cancer patients.
It found that Gleason 3 + 4 tumors with cribriform growth are associated with unfavorable outcomes and metastasis, while Gleason 3 + 4 without cribriform growth showed similar clinical behavior to Gleason 3 + 3.
For clarification, Howard Wolinsky posed the following question to Dr. Ming Zhou, Director of Urological Pathology at Mount Sinai Hospital in New York.
Do men with Gleason score 3+4 (grade group 2) prostate cancer without either cribriform changes or intraductal carcinoma have clinical outcomes comparable to men with Gleason score 3+3 (grade group 1) disease?
Dr. Zhou said,
Gleason pattern 4 is still considered a risk for progression compared to Gleason score 3+3 (Grade Group 2). Furthermore, increments in percent of Gleason pattern 4 correlate with increased risk for adverse clinical outcomes.
In other words, having no cribriform or IDC appears to “downgrade” the aggressiveness of Grade Group 2 cancer but does not make it entirely equivalent to Grade Group 1 cancer in terms of metastatic risk or cancer–specific mortality. That is why NCCN classifies Grade Group 1 as very low and low risk, and Grade Group 2 as intermediate risk.
An article about prostate cancer prognostic factors published in 2022 opined that the presence of cribriform growth pattern and intraductal carcinoma of the prostate should exclude Gleason 3 + 4 patients from AS.
However, challenges include inconsistencies in defining and reporting cribriform morphology on pathology reports and low detection on prostate biopsies due to sampling issues.
Prevalence of AS
A cohort study published in 2022 reports that U.S. rates of active surveillance increased sharply from 26.5% in 2014 to 60% in 2021."
MUSIC's AS program is likely the most successful in the United States. Howard Wolinsky reports that "it [currently] leads the country in putting low-risk patients on AS with a 91% rate."
Europe had a significant head start, with one study showing 85% of men in the Netherlands diagnosed in 2015-2016 with very low-risk prostate cancer were on AS. Another study showed that 91% of Swedish men diagnosed with very low-risk prostate cancer in 2014 were on AS.
Safety of AS
Active surveillance of low-risk prostate cancer, when done correctly, is very safe. Since 2015, several prospective protocol-managed AS cohort studies have published their long-term results showing 10- to 15-year metastasis-free and prostate cancer-specific survival rates ranging from 95–100% for low-risk prostate cancer.
Active Care
Prostate cancer centers of excellence use protocols that mostly follow NCCN guidelines. However, as these are guidelines and not rules, these centers are free to tweak their protocols to benefit both practitioners and patients.
For example, Johns Hopkins developed a software tool called Active Care to help prostate cancer patients on AS better understand their condition and help physicians make personalized care decisions.
The Active Care software tool:
Uses a prediction model developed by Johns Hopkins researchers based on decades of active surveillance data
Displays personalized predictions with clear data visualizations
Charts a patient's past lab results, MRI values, and the likelihood of finding a higher grade cancer on biopsy
Shows the probability of each cancer grade and long-term outcomes should a patient have his prostate removed
Helps patients better understand their risks and enables more confident decision-making about remaining in the active surveillance program vs pursuing treatment
Johns Hopkins says that since they deployed this software in 2016, AS team members have used it to make data-driven decisions regarding whether a patient needs to undergo a biopsy or seek treatment.
"Previously, patients were recommended to get a biopsy annually, but with the tool, most patients can wait, sometimes years longer, before undergoing the procedure."
Two significant issues in AS
The fact that patients can safely wait years before another prostate biopsy is emerging as a critical topic in AS. Men do not want digital rectal exams or prostate biopsies, and some will drop out of AS for those reasons, only to show up years later with advanced prostate cancer.
So, as testing and AS guidelines evolve, physicians and researchers should create and use evidence-based software tools like Active Care to decrease the amount of digital rectal exams and prostate biopsies that men undergo while on AS. In addition, optimizing artificial intelligence in AS programs will likely lead to safer and more effective programs with higher retention rates.
Overdetection
Researchers and practitioners should continuously reassess AS guidelines and protocols to find more ways to avoid overdetection of low-grade, low-risk prostate cancers that won't progress. As I discussed in a prior newsletter, Prostate Cancer Screening Part 2 - 016, overdiagnosis, also known as overdetection, is a big problem.
As more evidence-based tests emerge to augment prostate-specific antigen (PSA) testing, practitioners can use them to avoid unnecessary prostate biopsies. As Eric Kim, M.D., a urologist at the University of Nevada, Reno School of Medicine, states,
"...the ideal thing would be to have a non-invasive tool tell you as much as you can, and then you could really restrict the biopsies to the people that you think would benefit from knowing that they have prostate cancer."
Dr. Kim studies AI in prostate cancer and says that this ideal non-invasive tool will likely combine blood, urine, imaging, and AI to tell you who needs a biopsy and who doesn't.
Unnecessary rectal exams
As Howard Wolinsky, the writer of this Active Surveillor substack newsletter, has reported before, there is evidence coming from countries outside the U.S. that digital rectal exams aren't able to detect early prostate cancers and may be unnecessary.
Movember is an Australian men's health charity that funds prostate cancer research. It commissioned a report to gauge expert consensus on best practices and research priorities in active surveillance, which was published online in European Urology Oncology.
The consensus "agreed that best practice includes the use of high-quality magnetic resonance imaging, which can allow digital rectal examination and some biopsies to be omitted."
The concept of omitting unnecessary digital rectal exams and prostate biopsies is groundbreaking and hopefully foreshadows future expert guidelines in the U.S. This would not only help avoid overdiagnosis but would improve patient retention in AS programs.
Expert discussion
In 2020, urologist Matt Cooperberg, M.D. of the UCSF Comprehensive Cancer Care Center, interviewed two pioneers in active surveillance for prostate cancer - Peter Carroll, M.D., prior Chair of Urology at UCSF, and Laurie Klotz, M.D., Chief of Urology at the Sunnybrook institute at the University of Toronto.
Both interviewees said that their interest in active surveillance began after seeing a recurring pattern of overdetection and overtreatment of low-grade prostate cancer. Both agreed that active surveillance for men with Gleason 3 + 3 = 6 is a no-brainer.
However, caution should be taken in men with Gleason 3 + 4 = 7 to ensure that the pathologist's grading is accurate and that pattern four has a low volume. Dr. Carroll says, "It's actually volume more than grade that predicts outcome for 3 + 4 patients…" and recommends MRI and genomic testing to interrogate those with more volume on biopsies.
Dr. Klotz noted that before MRI and biomarkers, he observed that of the one hundred twenty 3 + 4 men he had been following on active surveillance over fifteen years, 30% developed metastases. "Way too high," he said.
He says surveillance for 3 plus small amounts of 4 is reasonable but recommends these men be checked with genomic testing and MRI if they are to remain under active surveillance. He says that while accurate imaging, including micro-ultrasound, makes a considerable difference, imaging is imperfect and misses significant cancers.
Klotz also says he doesn't promote genetic testing for men with very low-risk to low-risk disease, especially if they have favorable imaging and favorable targeted biopsy results. He feels genetic testing is not cost-effective in those men.
Dr. Carroll says that biomarkers appear to be better predictors than MRI for multivariable analyses but that no biomarker absolutely predicts or excludes progression. He recommends that these studies be used in context to consider an earlier confirmatory biopsy.
Dr. Coppersberg mentions that data from the Michigan Urologic Surgery Improvement Collaborative (MUSIC) and elsewhere show that many men initiated into active surveillance don't complete the minimal requirements.
Dr. Carroll says that to avoid this scenario, "We clearly need to make surveillance less intense for patients," and refers to reducing the frequency of biopsies.
Dr. Caroll mentions that good predictors of nonprogression include:
low PSA density
low PI-RADS scores
favorable genomic scores
a negative biopsy - up to 20% of men on active surveillance will have a follow-up negative prostate biopsy
He says these are factors to consider when following men on active surveillance less intensely.
Dr. Klotz agrees and says that MRI is replacing some of the biopsies and that, in his program, "We're now at about a five-year biopsy interval for the average patient, and I think you have to individualize this."
Dr. Copperberg asks if they think we'll ever get to a point where a low-volume single-core biopsy showing 3 +3 will be labeled something other than cancer.
Dr. Carroll replies, "I don't think so, actually. We've shown that even some people who have repeated negative biopsies can progress in the future. I think the public has to understand that cancer is a disease of increments…"
Dr. Klotz says, "…the nomenclature lies with the pathologist, so they're the ones we have to convince…" He says he's talked to people all over the world, and they're not ready to abandon the word cancer.
The future
Active surveillance of prostate cancer is evolving as researchers longitudinally study databases involving men on AS, such as Johns Hopkins' database, using their Active Care software tool. The technology is proprietary, but they should share the clinical findings with other researchers to create best practices for AS programs worldwide.
Databases containing patient characteristics, biomarkers, pathology slides, and imaging studies combined with artificial intelligence will create AS protocols that safely reduce overtreatment and optimize retention rates.
Creating programs that involve primary care providers and community-based urologists will help ensure prostate cancer patients on AS receive the recommended follow-up testing. Providing adequate education to patients and practitioners is critical in supporting adherence to AS protocols.
In addition, men with prostate cancer and their loved ones need adequate psychological support so they can make informed decisions in a supportive environment. The psychological burden of AS and fear of rectal exams and prostate biopsies is something practitioners need to factor in each time they interact with patients on AS.
A good motto for this might be, "Put yourself in their shoes." I think that's what the 2023 Movember consensus panel did when deciding that the use of high-quality MRI can safely reduce rectal exams and prostate biopsies for men on AS.
I can guarantee that the U.S. will lag other developing countries in this regard. Why? Because we've already seen this pattern in the adoption of AS compared to European countries.
Incentives are necessary
Why did MUSIC's AS program take the lead in adopting AS in the U.S.? One reason is a financial incentive. As reported in the Urology Times Journal,
"When the collaborative meets specific performance targets, participating urologists receive higher reimbursement (up to 105% of standard fee) from Blue Cross Blue Shield of Michigan (BCBSM)."
Doctors, for the most part, have the patients’ best interests at heart. But what sustains the incentive to always do the right thing? Higher reimbursement rates and feedback on meeting AS practice parameters.
It's too bad we can't create that kind of collaboration nationwide because it's already proven to work in one state. If Michigan can do it, so can everyone else.
I wish all of you the very best.
Keith R. Holden, M.D.